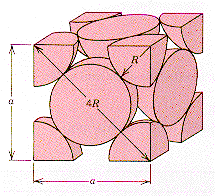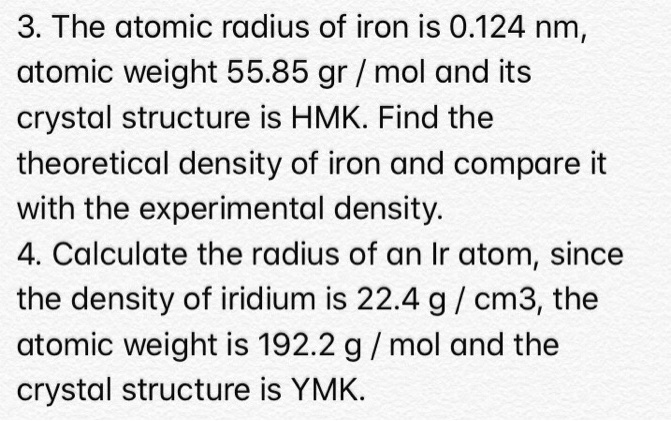
SOLVED: The atomic radius of iron is 0.124 nm, atomic weight is 55.85 g/mol, and its crystal structure is HCP. Find the theoretical density of iron and compare it with the experimental

Ionic radius and electron configuration for (a) Fe; (b) Fe +2 and (c)... | Download Scientific Diagram
38. The effective radius of iron atom is 1.42 Angstrom. It has rock salt like structure,Calculate its density(Fe=56smu)?

OneClass: Iron has a BCC crystal structure, an atomic radius of 0.124 nm, and an atomic weight of 55....

Solved) - Rhodium has an atomic radius of 0.1345 nm and a density of 12.41... (1 Answer) | Transtutors

The effective radius of iron atom is √2˚A. It has FCC structure. Calculate its density (Fe=56amu,NA=6×1023)