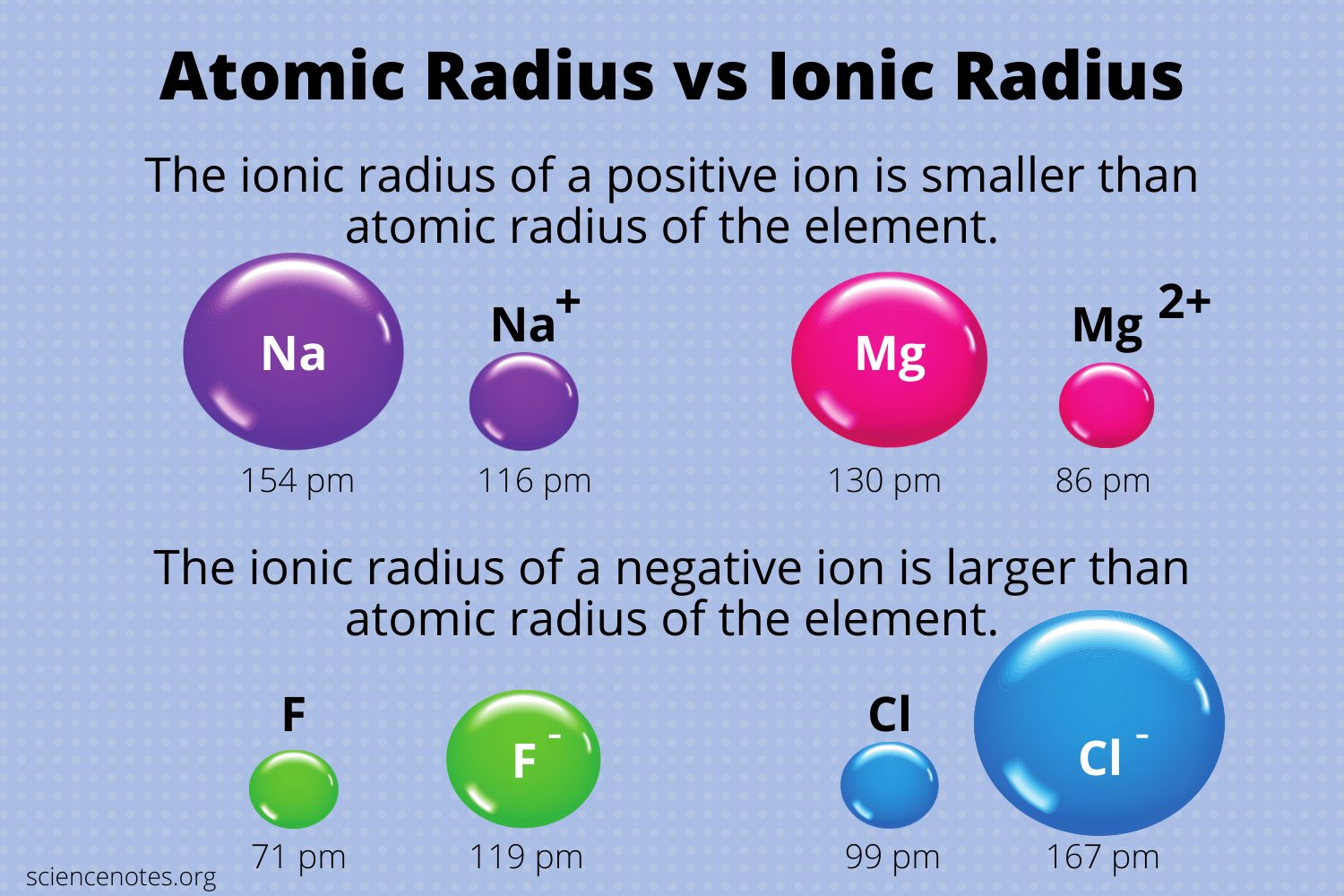
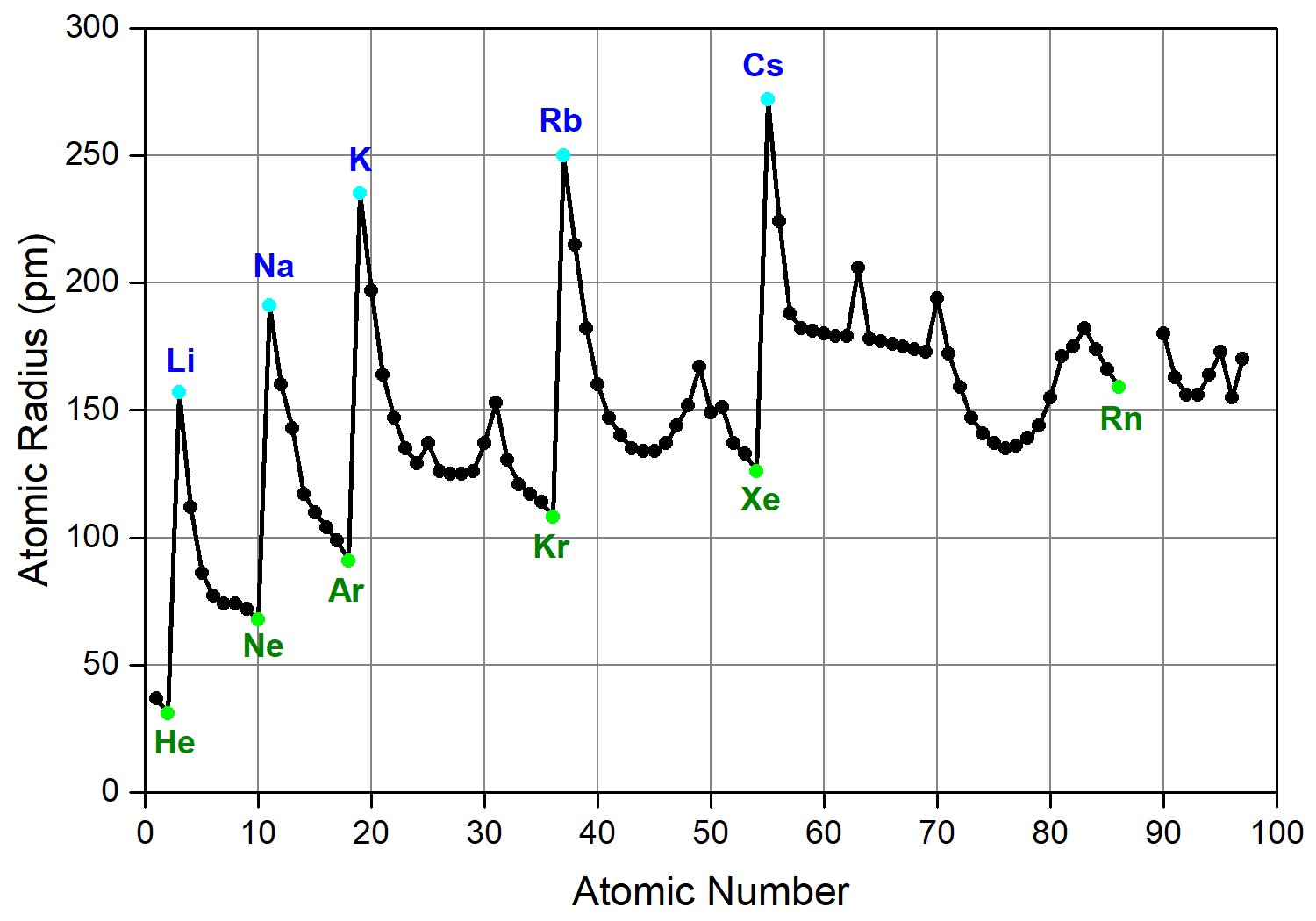
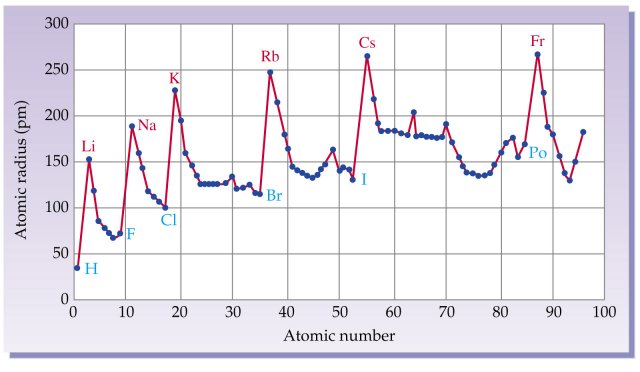
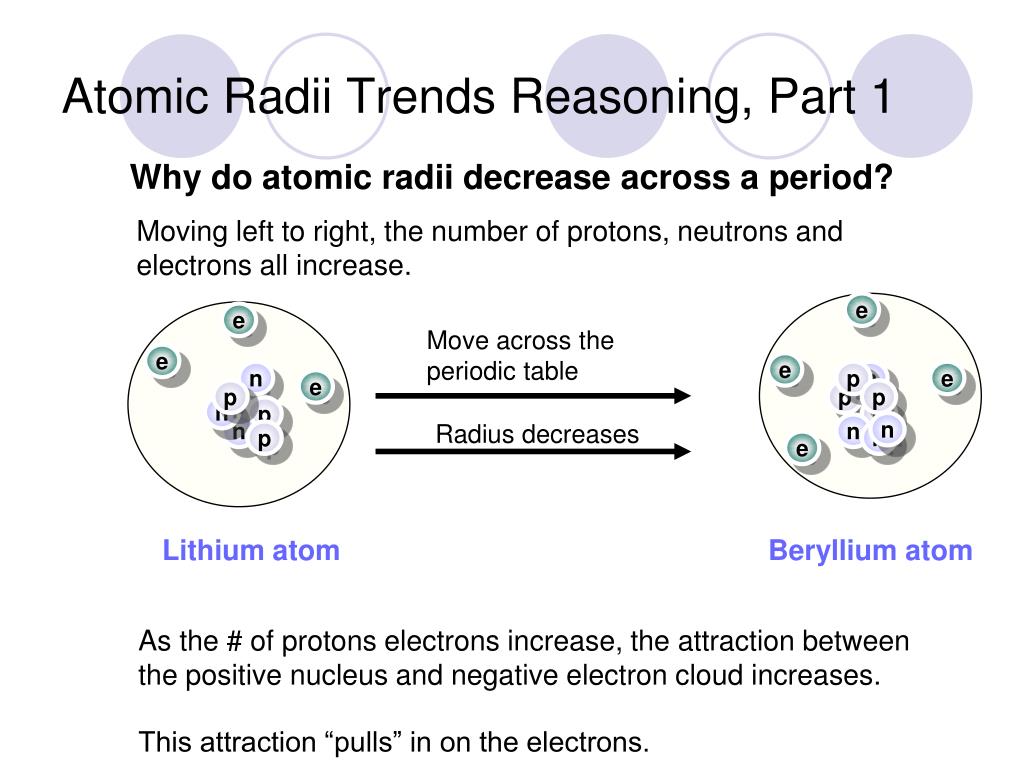
Why does atomic radius decrease for Period 4 elements (Ti, V, Cr, Mn) and then increases for Fe, Co, Ni, Cu? - Quora
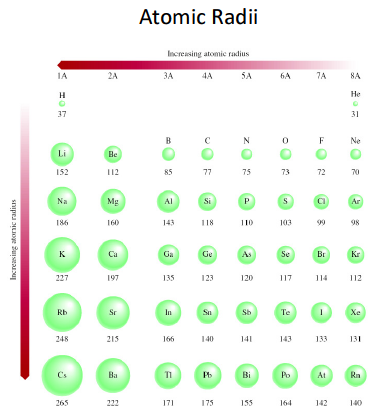
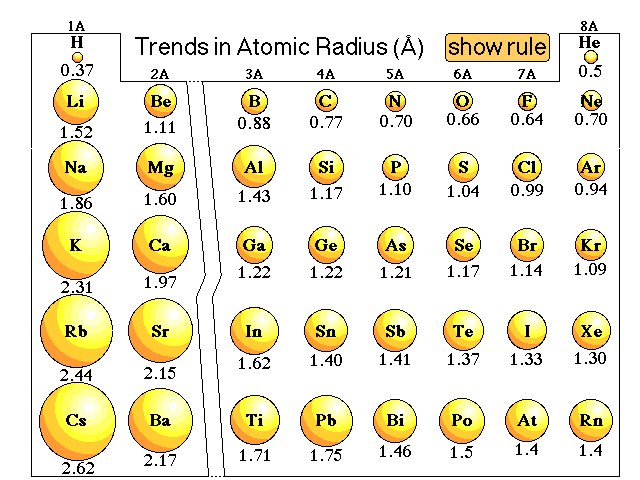
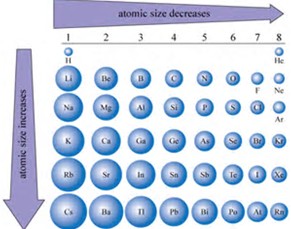
Why does the atomic radius of an element generally decrease across a period and why does it increase down a group? | Socratic

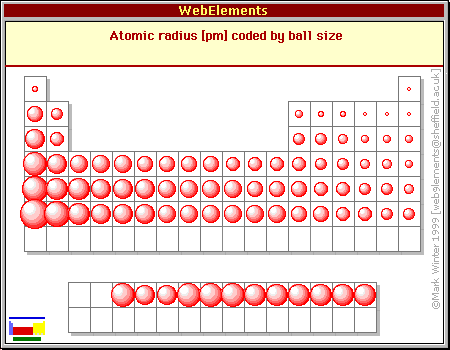
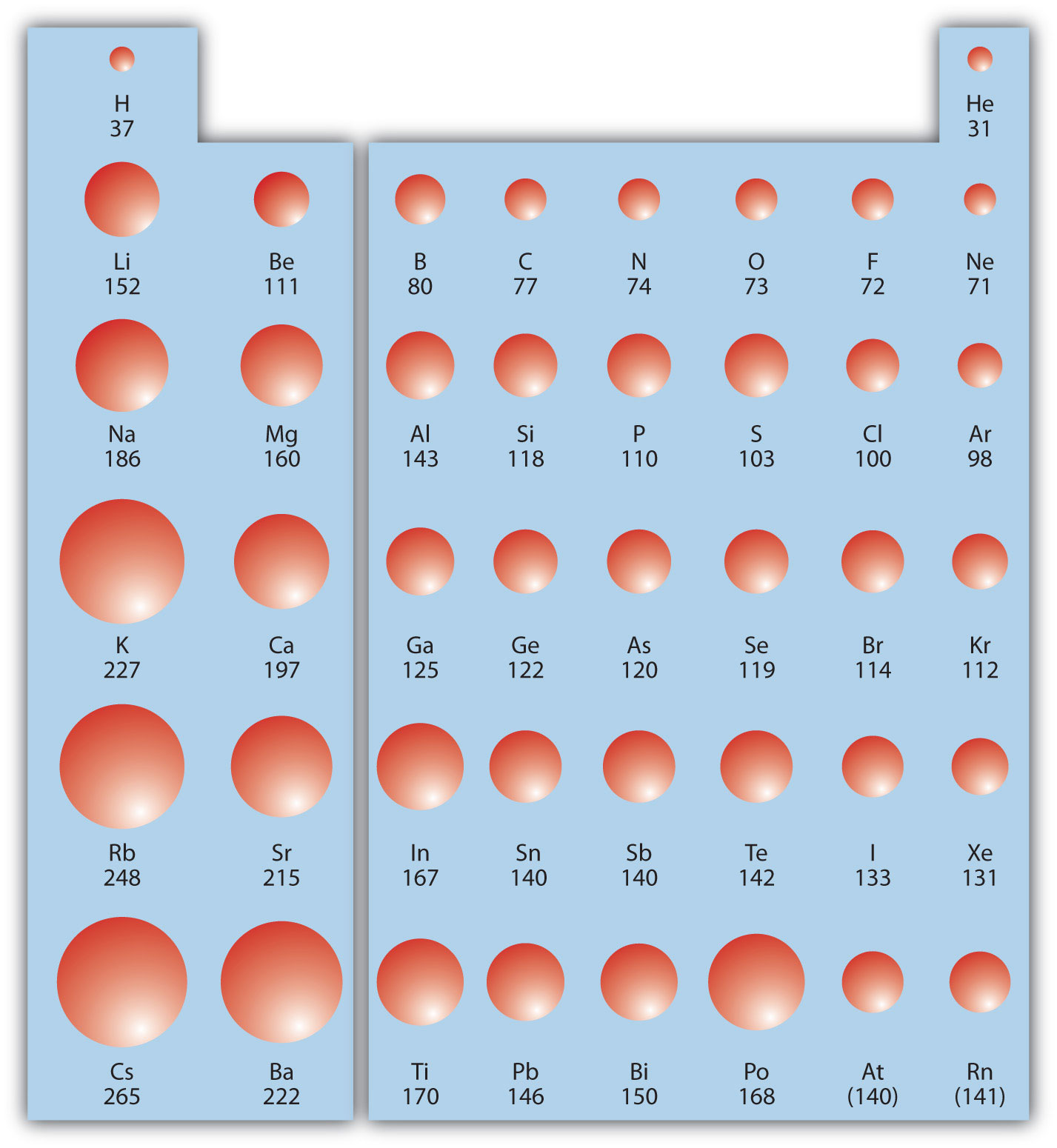
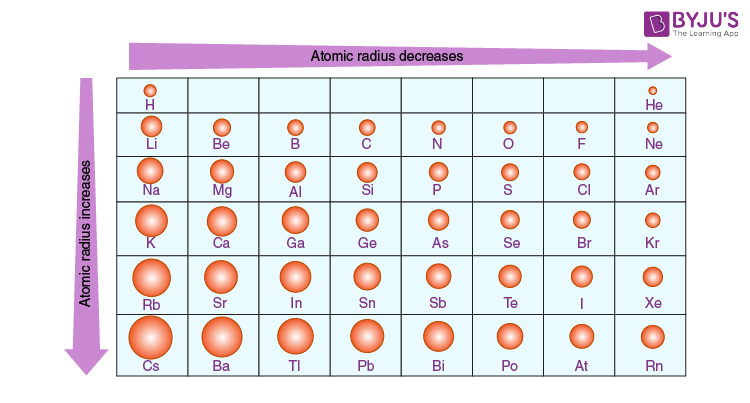
Trends in the Periodic Table (Chpt. 7). 1. Atomic radius (size) 2. Ionization energy 3. Electronegativity The three properties of elements whose changes. - ppt download

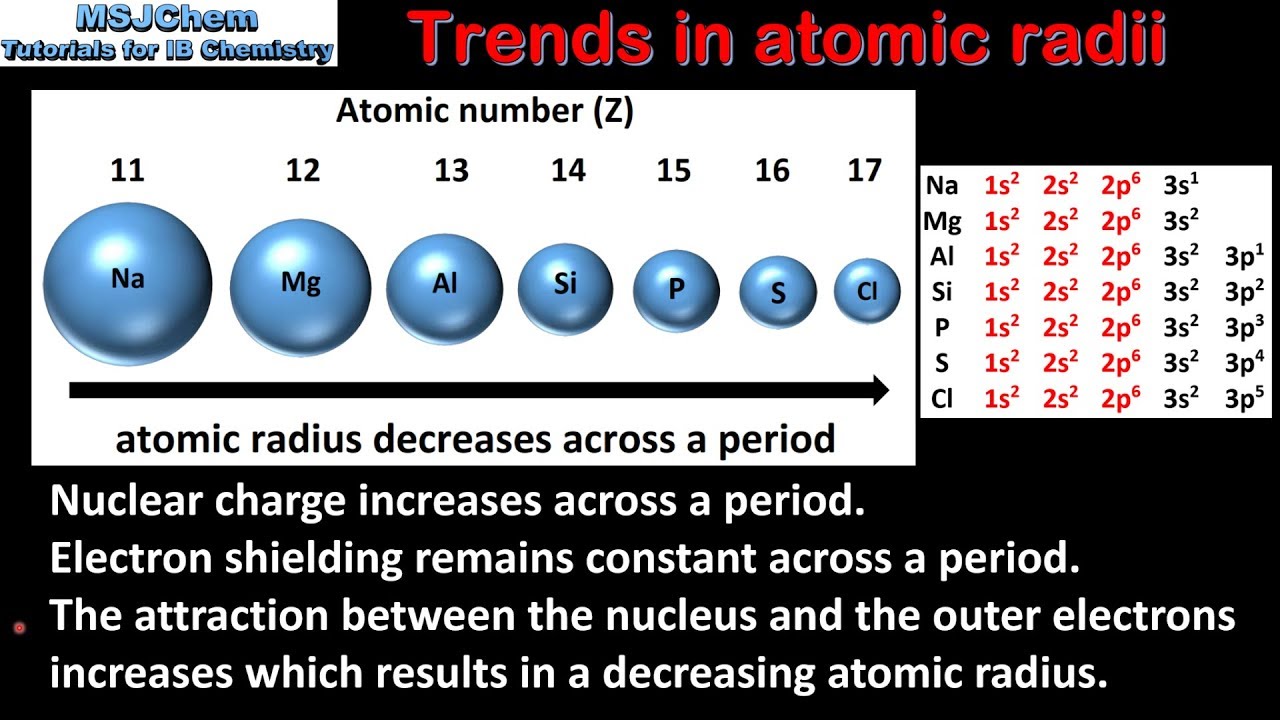
Why does the atomic radius generally decrease across a period (from left to right)? | Homework.Study.com